1. Introduction
Multiple sclerosis (MS) is an inflammatory disorder that affects the central nervous system that affects more than 2 million individuals globally and it primarily affects young people [1], [2], [3]. Although its cause is unknown, the pathogenic process appears to begin with an autoimmune attack on myelin components [4,5]. Loss of axons in the central nervous system and also the inflammatory reaction to this loss have only recently been fully identified [6,7]. Treatments for multiple sclerosis involve a cooperative effort between the body's immune system and the drug to reverse the damage caused by demyelination [8]. Unfortunately, conventional treatments for multiple sclerosis, like Glatiramer acetate and interferon beta, are only partially effective [9,10]. This is due to the inability of medications to generate or relocate sufficient quantities of brain stem cells, which would allow treatments to have a major effect on the immune system in cases of brain injury [11,12]. On the other hand, non-selective immunosuppression must be replaced with novel approaches of immune control and therapeutic interventions that enhance both immunomodulation and neuroprotection [13,14]. Recent research suggests that external variables, including as viral infections, may play a role in multiple sclerosis relapses [15,16]. The mechanism, which is likely complex, through which the disorder operates, is still a mystery. Unfortunately, immunosuppressive medicines are ineffective at halting the progression of MS, and newer therapy like fingolimod and dimethyl fumarate have not been shown to improve neurodegeneration in MS patients [17,18].. Newer drugs, such as ocrelizumab, and repurposed older drugs, such as cladribine, have proven to be highly effective therapies for relapsing MS. Patients who have not responded to other treatments may find new hope in HSCT because of the progress that has been made in the field, which has decreased the technique's morbidity and mortality. Although ocrelizumab has been shown to be effective in treating primary progressive multiple sclerosis (PPMS), and siponimod has shown promise in treating active secondary progressive multiple sclerosis (SPMS), and ibudilast and high-dose biotin have shown promise in treating progressive secondary multiple sclerosis, there is still a significant unmet need for strategies to repair or restore damaged neurons [19]. As a result, an increasing number of people are investigating cell replacement treatment as a method of reviving the body's capacity for myelin repair [20,21]. Neuroprotective mechanisms and the ability to generate new brain cells are potential benefits for MS patients receiving stem cell transplantation [22,23]. Since its start, the fundamental goal of regenerative therapy for multiple sclerosis has been to restore myelin (MS). The formation of new oligodendrocytes from OPCs may help in the remyelination process in animal models of multiple sclerosis [24,25].
So far, several molecular and cellular pathways involved in intrinsic brain repair have been discovered. There are essentially three distinct but interconnected groups. Brain repair is aided by two processes: (i) inflammation-driven processes, which help when humoral and cellular inflammatory components shift balance (function) from a tissue-damaging mode to a mode promoting tissue repair (e.g., neurotrophic support from inflammatory cells); and (ii) brain plasticity, which involves the recruitment of alternative "non-damaged" functioning neuronal pathways (cortical maps) primarily via axonal branching and synaptogenesis [26,27]. Endogenous adult neural stem/precursor cells, if they survive the inflammatory and/or degenerative action, may migrate within damaged areas and promote repair via multiple mechanisms of action, including cell replacement, remyelination, and/or neuroprotection, thereby helping to maintain brain plasticity [28,29]. Many researchers are still unsure if stem cells of a different embryonic origin may help with brain repair or not, in addition to brain stem cells. Several functions, including as cell replacement by trans-differentiation, fusion, and immunosuppression, have been proposed for these cells. However, more research is needed to establish the extent to which these cells contribute [30,31].
Remyelination can be shown in over 40% of MS lesions, suggesting that healing of the brain occurs spontaneously in MS [32]. Remyelination is promoted by oligodendrocyte precursor cells (OPCs), but there is evidence to suggest that OPCs are not the only cell type involved in this process in multiple sclerosis [33,34]. Adult neural stem and precursor cells (hereafter referred to as adult neural precursor cells or NPCs) may also play a role in remyelination because of their role in supporting neurogenesis and gliogenesis [35].
In contrast to the inefficiency of neuronal regeneration, mature mammalian central nervous systems are exceptionally good at regenerating myelin. The remyelination of the central nervous system is carried out by adult oligodendrocyte precursor cells, which are a subset of neural stem/progenitor cells (OPCs) [36]. More than 5% of the adult CNS is made up of progenitor cells that can develop into oligodendrocytes, Schwann cells, or neurons. After central nervous system (CNS) demyelination, nearby adult OPCs can be induced to migrate to the damaged areas and develop into functional oligodendrocytes, which can then be used to repair the myelin sheath [37]. Effective remyelination is possible at the earliest stages of multiple sclerosis, and this may contribute to better functional recovery throughout the remission phase [38]. Axonal degeneration and functional deterioration accelerate in the latter stages of multiple sclerosis for unknown reasons, when remyelination is less effective [39]. OPCs can still be attracted to lesions in MS with chronic disease. They do not seem to be able to develop into oligodendrocytes intended to promote myelin repair [40]. As a result, it is critical to discover signaling pathways and pharmacological compounds that boost endogenous adult OPC differentiation in order to overcome the OPC differentiation block and enhance remyelination in MS. In addition, preserving the integrity of CNS axons is likely to be slowed or avoided altogether if myelin regeneration is enhanced [40].
The purpose of this article is to address the potential impact of the engineered neural stem cells and defining recommendations for the use of neural stem cell transplantation as a treatment option for progressive multiple sclerosis [41,42]. This article discusses multiple sclerosis and the potential for neural stem cell treatment to treat the disease [43,44]. It explores questions including how the cells get there, how the recipient's immune system responds, how NSCs communicate with the rest of the brain, and whether or not tumors can form as a result [45], [46], [47].
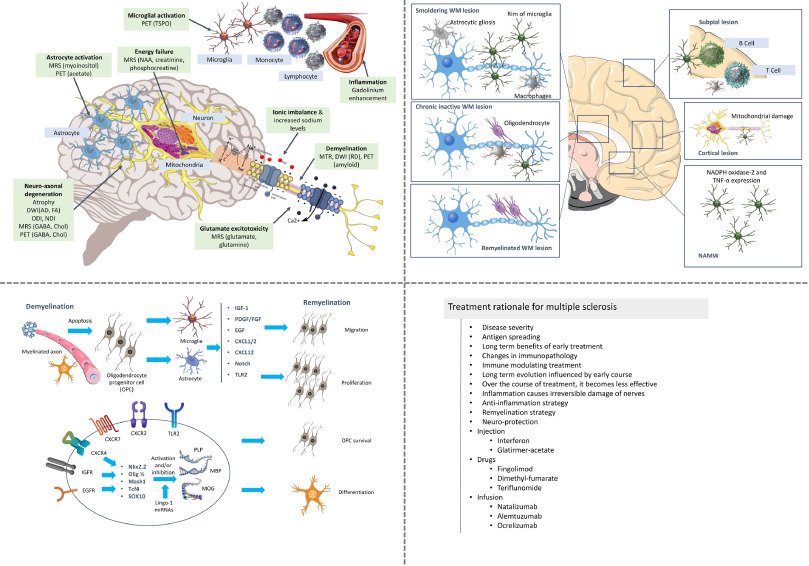
Fig. 1 illustrates the disease progression, therapeutic targets and the strategies that could be used in the treatment of multiple sclerosis. The following section will elaborate the neural stem discussing the background and the potential of its use in the therapy for multiple sclerosis.
 Fig. 1. The pathophysiology, therapeutic targets, treatment rationale and treatment strategies for multiple sclerosis [reproduced with permission from [3,48,49,50]].
Fig. 1. The pathophysiology, therapeutic targets, treatment rationale and treatment strategies for multiple sclerosis [reproduced with permission from [3,48,49,50]].2. Potential therapeutic value of neural stem cells in MS
Neuronal progenitor cells (NPCs) can be detected in both the embryonic and adult brain. They represent a heterogeneous group of proliferating, differentiated, and regenerative cells. Different genes are expressed at different times and in different places in these cells, and this spatial and temporal variability is highly complex. Newly formed neurons may have been among the proliferating neural cells discovered in the adult rat brain in the late 1960s. Since that time, NPC embryos have been housed in a separate facility from regular embryos and not just the adult brain and spinal cord. The ganglionic eminence(s), the subventricular zone(s) of the lateral ventricles, and the subgranular zone(s) of the hippocampal dentate gyrus (DG) in the adult have all been demonstrated to contain stem-cell-like progenitors that can promote neurogenesis and gliogenesis. These regions were later classified as central nervous system germinal niches. These cells are assumed to represent CNS stem cells because of their slow proliferation and immunoreactivity for glial fibrillary acidic protein (GFAP), nestin, and eventually the radial glia marker RC2. Rapid advances in technology have made it possible to safely expand and preserve CNS stem cells in chemically defined environments for years after their in vivodiscovery. These methods were developed after the in vivo identification of CNS stem cells. As a result, methods have emerged for producing a high volume of NPCs in vitro, which strengthens the potential of these cells to be used as a never-ending source of untainted, transplantable stem cells. Stroke, Parkinson's disease, Huntington's disease, multiple sclerosis, and spinal cord injury are only some of the neurological illnesses and traumas that have benefited from NPC-based treatments (SCI). Many of these attempts have been successful in animal tests, but many challenges remain before these medications may even be considered for use in people. Which type of cell (embryonic or adult) and which route of administration (injection, infusion, etc.) is optimal for transplantation is still up for debate (local or systemic). Nonetheless, the mechanisms that might keep NPCs functioning normally after transplantation and keep wounds from healing over time are not well-established at this time. There is insufficient evidence to support the idea that transplanted NPCs may successfully reconstruct the 3D brain architecture and generate a sufficient number of fully functioning cells capable of integrating into brain circuitries. Stem cells have the potential to move to the appropriate site and differentiate into the needed cell type, but this is not always sufficient.
Restoring the endogenous stem cell compartment via transplantation of functional NPCs is a potential treatment for multiple sclerosis due to the dysfunction of brain germinal niches in this disease. Due to the presence of a hostile inflammatory environment in the major germinal niches of MS brains, restoration would be required in ectopic CNS regions. It has been demonstrated that therapeutic somatic stem cells [e.g., induced pluripotent stem cells] are effective in treating multifocal inflammatory CNS disorders (such as stroke, multiple sclerosis, brain tumors, spinal cord injury, epilepsy, brain trauma, amyotrophic lateral sclerosis, etc.). If injected into the bloodstream or cerebrospinal fluid circulation, bone marrow stem cells (BMSC), umbilical cord blood stem cells (UCBSC), mesenchymal stem cells (MSC), and neural progenitor cells (NPC) can reach inflammatory CNS regions where they stay for months and promote recovery. How mitosis happens selectively in these cells during the cell cycle Neuronal cells have recently been demonstrated to target inflamed CNS regions in animals with chronic or relapsing-remitting EAE. The drug was administered intravenously and intracerebroventricularly to mice. In order to restore neurological function, NPCs are directed to inflammatory regions of the central nervous system (CNS), where they reduce demyelination, axonal loss, and astrogliosis significantly. NPCs exhibit constitutively high-affinity cell adhesion molecules (CAM), which include CD44, integrins, and functional chemokine receptors (CCR1, CCR2, CCR5, CXCR3, and CXCR4). These chemicals enable NPCs to employ the same routes into the CNS as inflammatory immune cells. In fact, CAM-expressing NPCs can respond to the generation of local cytokines (such as IL-6, IL-1, and TNF-) in CNS regions. They spontaneously attach and move to the irritated endothelium following inflammation. In an in vitro experiment, scientists used neutralizing antibodies against 4 integrin to reduce the quantity of injected NPCs by 50–60%.
And the spinal cord of mice with EAE infection. When systemically injected, NPCs aggregate (and persist) in the perivascular space of the central nervous system (CNS) alongside inflammatory cells such as reactive astrocytes, inflamed endothelial cells, and invading T lymphocytes. There is molecular cross-communication between the cells in these atypical niches, also known as "CNS atypical ectopic niches." Due to the localized release of stem cell regulators (e.g. BMPs, Noggin) by immune cells and stromal cells, the vast majority of transplanted NPCs display undifferentiated characteristics (e.g., round-shaped morphology and absence of key markers of differentiation).
Activated astrocytes are present. Instead, NPCs promote neuroprotection by inducing apoptosis. Locally released substances include neurotrophic factors such as nerve growth factor (NGF), fibroblast growth factor (FGF)-II, ciliary neurotrophic factor (CNTF), and brain-derived neurotrophic factor (BDNF), as well as immunomodulatory compounds such as anti-inflammatory cytokines. NPCs stimulate the apoptosis of effector cells with death receptors (such as encephalitogenic Th1 cells) via releasing or generating immunomodulatory substances (such as FasL, Apo3L, TRAIL). NPCs drastically diminish glial scarring via neurotrophic growth factor release (such as TGF FGF-II). In conclusion, NPC transplants may develop into myelin-forming cells. The combined result of these various neuroprotective activities was a fivefold increase in remyelinating areas in demyelinating regions. Table 1 will list the cell sources, identification, and the culture method of neural stem cells. Table 2 discusses the cell source, factors involved and the effect of immune cells on the neural stem cells.
Table 1. Neural stem cell sources, identification, and culture method.
| SL No. | Source of NSC | Identification | Culture method | Refs. |
|---|---|---|---|---|
| 1. | Bone marrow & adipose tissue derived mesenchymal stem cells | Nestin | Neurospheres, epidermal growth factor, fibroblast growth factor supplemented media | [51,52] |
| 2. | Embryonic stem cells | Nestin | Embryoid bodies | [53,54,55] |
| 3. | Embryonic neural stem cells | Nestin | Neurospheres, epidermal growth factor, fibroblast growth factor supplemented media | [7,56] |
| 4. | Human fetal nervous system | Nestin | Neurospheres, epidermal growth factor, fibroblast growth factor supplemented media | [41,57,58] |
| 5. | Human adult nervous system | Glial fibrillary astrocyte protein & vimentin | Neurospheres, epidermal growth factor, fibroblast growth factor supplemented media | [5,59] |
| 6. | Human postmortem brain | Glial fibrillary astrocyte protein, nestin, Sox-2, CD133 | Neurospheres, epidermal growth factor, fibroblast growth factor supplemented media | [60,61] |
| 7. | Endogenous subventricular zone | Nestin, LeX, CD133 | None | [55,62,63] |
| 8. | Exogenous skin | CD133, nestin | Neurospheres, epidermal growth factor, fibroblast growth factor supplemented media | [51,52] |
Table 2. Mechanisms influencing immune cell impact on neural stem cells.
| SL No. | Factor | Cell source | Effect | Refs. |
|---|---|---|---|---|
| 1. | VEGF-α | Endothelial cells, astrocytes | Increase in proliferation and migration | [64,65] |
| 2. | PDGF-α | Neurons & astrocytes | Increase in proliferation and migration | [66,51,67] |
| 3. | TNF-α | Macrophages & microglia | Decrease in proliferation | [68,52] |
| 4. | IFN- α | Neurons, endothelial cells, macrophages, dendritic cells | Decrease in proliferation | [63] |
| 5. | CCL5 | Microglia, macrophages, lymphocytes, astrocytes | Increase in proliferation | [69,70] |
| 6. | TGF-β | Neurons, macrophages, astrocytes, microglia | Increase in differentiation | [53] |
| 7. | IGF-1 | Blood mononeuclear cells | Increase in differentiation and proliferation | [71,72] |
| 8. | GDNF | Blood mononeuclear cells | Increase in survival and differentiation. | [73,54] |
| 9. | BDNF | Microglia, lymphocytes, monocytes, astrocytes | Increase in survival and differentiation. | [7,74,75] |
| 10. | CNTF | Astrocytes | Increase in survival and differentiation | [76] |
| 11. | IL-10 | Macrophages, microglia, astrocytes, lymphocytes | Increase in migration | [77,78,58] |
| 12. | IL-15 | Microglia | Increase in proliferation | [10,79] |
| 13. | IL-6 | Macrophages, microglia, astrocytes, lymphocytes | Decrease in proliferation | [80,81] |
| 14. | IL-4 | Macrophages, T cells, Microglia | Increase in migration and differentiation | [18,57,82] |
| 15. | CXCL12 | Astrocytes, endothelial cells, meningeal cells | Increase in migration | [83,59] |
| 16. | CCL11 | Macrophages, microglia, lymphocyte, astrocyte | Decrease in proliferation and differentiation | [11,84,85] |
| 17. | CX3CL1 | Macrophages, microglia, lymphocyte, astrocyte | Increase in proliferation | [2] |
2.1. NSC survival, differentiation and immunomodulation ability
Microglia are monocyte-derived cells that have a role in the nervous system [5,86]. These proteins may have a function in the early and late phases of multiple sclerosis [8]. Proinflammatory chemicals cause microglia clusters to form, which then release reactive oxygen and nitrogen species into the surrounding environment, causing inflammation (NO). As a result, demyelinated axons have more energy imbalance [18,24].
Myelin repair requires microglia to remove damaged myelin. The cytokines released by oligodendrocytes in response to inflammation and infection attract microglia to phagocytosis inhibitory substances, which impede phagocytosis [66]. When latent SPMS lesions were awoken, microglia were activated. Researchers have demonstrated that microglia are not capable of phagocytosing significant amounts of myelin [87,73].
Microglia have been found to reduce CNS inflammation when given to MS patients (MS) [88,89]. In Multiple Sclerosis, this can be harmful to oligodendrocytes because it leads to the production of proinflammatory mediators such chemokines and free radicals [41,43].
Microglia have been linked to NSC migration, survival, and differentiation in a number of studies [68,90]. The researchers discovered that NPC recipients had much larger numbers of microglia in both acute and chronic EAE than the general population [91]. The soluble compounds released by mouse microglial cells aid NPC migration in both laboratory and natural settings [[92], [93], [94]].
It is hypothesized that modulating the phenotype and activity of host microglia will aid in neuroprotection and regeneration in the brain [44,95]. Microscopic immune cells called microglia not only generate but also control the behavior of brain stem cells (NSCs). In the adult mouse brain, neuronal stem cells (NSCs) have been demonstrated to be neuroprotective through the production of neuroprotective chemicals [96]. It has been shown that the neuroprotective molecule CX3CR1, produced by microglia, aids mice with neurological disorders (TREM2) [80,71]. After NPC transplantation, the number of Iba-1+ activated microglia in the striatum of mice (C57BL/6) goes up, which is in line with what has been found in the past [47]. NSCs promote host neuron survival in mouse organotypic brain slice cultures by activating the microglial TLR9-ERK1/2 pathway [97,98]. The TLR9-ERK1/2 pathway in microglia is altered. By blocking NSC regulation, preconditioning with the microglial inhibitor minocycline enhanced graft survival and paracrine mediator synthesis in the laboratory [[64], [99], [100]]. In those with persistent EAE, microglial activation increased the SVZ's ability to regenerate [84]. Because minocytic cells were reduced, NSCs could multiply and grow into oligodendrocytes [[83], [101], [102]]. Fig. 2illustrates the treatment strategies for multiple sclerosis using neural stem cells. Table 3 lists the infusion of the neural stem cells into different animal models for the treatment of multiple sclerosis. The next section will discuss the route of administration of the neural stem cells.
 Fig. 2. Treating multiple sclerosis using neural stem cell engineering, This illustrates the sources, differentiation, implantation and the treatment strategies of neural stem cell in multiple sclerosis therapy [reproduced with permission from [103,104,105,106,107]].
Fig. 2. Treating multiple sclerosis using neural stem cell engineering, This illustrates the sources, differentiation, implantation and the treatment strategies of neural stem cell in multiple sclerosis therapy [reproduced with permission from [103,104,105,106,107]].Table 3. Infusion of NSPCs into experimental animals of multiple sclerosis.
| SL No. | Disease | Source | Model | Outcome | Route | Differentiation | Other | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1. | Multiple sclerosis |
|
Chronic EAE mouse | Improvement in locomotor activity | Intravenous | No neural or glial differentiation | LIF mediated inhibition of encephalitogenic TH17 cell differentiation | [66,108] |
| 2. | Multiple sclerosis |
|
Chronic EAE mouse | Improvement in locomotor activity | Intracerebroventricular & Intravenous | Oligodendroglial & neuronal differentiation | Inhibition of peripheral and CNS confined inflammation. | [22,109,110] |
| 3. | Multiple sclerosis |
|
Chronic EAE mouse | Improvement in locomotor activity | Intracerebroventricular & Intravenous | No neural or glial differentiation | Inhibition of dendritic cell activation and lymphatic proliferation | [68] |
| 4. | Multiple sclerosis |
|
Relapsing EAE Mouse | Improvement in locomotor activity | Intravenous | Untested | Induction of apoptosis of CNS infiltrating T lymphocytes | [73,54] |
| 5. | Multiple sclerosis |
|
Chronic EAE mouse | Improvement in locomotor activity | Intracerebroventricular & Intravenous | Oligodendroglial & neuronal differentiation | Modulation of neurotropic auditor growth factors | [10,18,111] |
| 6. | Multiple sclerosis |
|
Acute EAE rat | Improvement in locomotor activity | Intracerebroventricular | Untested | Inhibition of MOG-specific proliferation | [82,112] |
2.2. Route of administration of NSC
Due to the fact that NSCs are able to cross the blood-brain barrier, intravenous or intrathecal delivery is the more effective method [113,114]. No central nervous system damage was observed following the subcutaneous or intravenous introduction of NPCs to mice with EAE (Experimental autoimmune encephalomyelitis) [115]. NPCs were found in lower numbers in the central nervous system and secondary lymphoid organs. Because the damage to the central nervous system is multi-focal, chronic, and geographically distributed, targeted NSC injections are not a viable therapy option for multiple sclerosis (MS) [57,116]. Multifocal lesions require a lot of local injections [111]. The incapacity of transplanted NSCs to travel long distances inside the CNS parenchyma after transplantation may hinder intrathecal delivery [84,72].
Injections of NSC into the central nervous systems (CNS) of mice and rats were investigated both directly and subcutaneously by the researchers [117,118]. Based on research NPCs transplanted into newborns successfully differentiated into oligodendrocytes, demonstrating their potential for restoring function after white matter inflammation (but not in nearby gray matter areas) [3,119].
Intranasal administration of NSCs is a painless and safe way to treat patients. Nasal stem cells (NSCs) can go to the central nervous system (CNS) of EAE mice and aid in functional recovery [[59], [120], [121]]. Researchers found that injecting NSCs into mice's carotid arteries improved cell homing and behavioral recovery [122]. The EAE analysis found no evidence of carotid NSC delivery [77,123]. Pathogenic immune cells appear to have a closer relationship with peripheral immunity than invasive pathogenic immune cells. The NSCs in the central nervous system (CNS) should be more efficient than those in the peripheral nervous system (PNS) at taming inflammation (PNS) [124,125].
2.3. Risks associated with the use of NSC
To date, there have been insufficient long-term follow-up studies or in-depth investigations of the potential risks of therapeutically employing adult brain stem cells [57,126]. Not only from an ethical standpoint, but also from a technological one, and because the patient's life is on the line [127]. These dangers are associated with most of the treatments discussed below, such as: It's possible that a tumor will develop. Teratomas have been discovered in the striatal cortex of experimental mice with Parkinson's disease by multiple researchers [[48], [128], [129]]. In one research, undifferentiated stem cells constituted 20% of all new cancers [130]. Using viral vectors or genetic modifications from regulator genes to promote dopaminergic neuron growth and efficacy may result in uncontrolled viral transmission and mutagenesis [131,132]. Stem cells may be harmful in and of themselves. Extending culture time, as with embryonic stem cells, enhanced genetic and epigenetic changes [113,114]. In the white matter, sub-ependymal region, and cortical gray matter, there is a lack of migration and the presence of heterotopias [7,115,133]. Heterotopias occur when cells migrate outside their intended location, resulting in refractory epilepsy or other neuropsychiatric illnesses [111,72]. Despite great progress, the mechanisms of adult neural stem cell differentiation and target migration remain unclear [21,134]. Adult neural stem cells require more immunosuppression (cyclosporine) than embryonic mesencephalic stem cells to avoid rejection and maintain the therapeutic response [30,118,135]. Adult tissues, with their highly differentiated and antigenic cells, may require immunosuppressive drugs [59,77]. However, these medications may cause liver and kidney damage, hypertension, and immunodeficiency in certain people [123]. There are risks with operation. But brain cell transplants are not without risk, even when they are done stereotactically with exact mapping and coordinates [60]. Surgery-related bleeding or infection risks are roughly 3% of all surgical risks [136,137]. Even though the risk is lower than deep brain stimulation with a foreign body, a thorough risk-benefit analysis is required [42,82]. Infections HIV/Aids, CMV, and herpes simplex virus can be transmitted from donor to recipient after cell transplantation [138]. Yeast infections, spore infections, and prion diseases are all possible in this environment [[53], [139], [140]].
The success of NSC transplantation primarily depends on the cell fate precommitment of transplanted NSCs into OPCs, while at the same time the endogenous differentiation of OPCs needs to be boosted in chronic stages of the disease [69]. There is some evidence from preclinical studies that NSCs and NPCs can activate the endogenous NSC pool and also exert an immunomodulatory effect. NSCs are aiming to regulate microglial activity in the central nervous system. However, microglia activity varies by MS stage, therefore optimal intervention time must be carefully investigated [66,83]. The whole scope of cellular renewal is currently unknown and should be investigated further. However, there are a few tricky problems that need fixing. It is necessary to first establish methods for large scale generation NSCs and NPCs from human iPSCs or by directly converting somatic cells into iNSCs. Engineering approaches of neural stem cells could be a viable option to produce personalized neural stem cells at a large-scale using biomaterials. The following section elaborates the engineering approaches of the neural stem cells [60,141].
3. Current NSC engineering approaches for MS treatment
Neural stem cells (NSCs) can be used to repair damage to both the peripheral and central nervous systems [132,142]. They have the potential as a treatment due to their ability to self-renew and differentiate into neurons and glia (astrocytes and oligodendrocytes) [113]. Injured tissue can benefit from NSC transplantation because they stimulate new nerve cell proliferation and axonal extension from the donor cells. There is no induction of NSCs by either the medium or the buffer [143]. Thus, NSC survival and development in vivo are hindered. Cellular activity can cause ischemia and inflammation, limiting the efficacy of NSC transplantation [114,144]. The inhibition of apoptosis may be beneficial to the growth of NSCs. It is impossible to fully repair injured nerves without the transplantation of NSCs [145]. Using functional scaffolds to transplant NSCs from ESCs and iPSCs appears to increase in vivo survival (iPSCs) [95,96]. To develop NSC microenvironments in vivo, biomaterial substrates and scaffolds must provide both physical support and matrix-cell interactions. These substrates and scaffolds can change stem cell activity [115]. There are a number of bioactives that promote cell adhesion, proliferation, and differentiation in neural stem cells (NSCs) [2,5]. Peptides and growth factors can be immobilized on surfaces through chemical or physical adsorption. Multi-step methods include surface activation and chemical functionalization [115,79,146]. Substrates used to cultivate NSCs are often chemically treated to activate growth factors. Bulk material characteristics, surface denaturation, and batch variability are all susceptible to these changes [4]. Surface conjugation can be inhibited by adding activators and immobilizers to surface functional groups (NHS and maleimide). As a result of their non-specific character, ECM proteins and peptides are not retained by physical adsorption [57,133]. Rapid immobilization of a bioactive culture substrate or biomimetic tissue scaffold is achievable [126,147].
Lesions and glial proliferation slow CNS tissue regeneration. After transplantation, the donor cells seem to generate random patterns around the lesion [148]. It is uncertain how the transplant interacts with normal brain tissue neurons to form networks. Tissue engineering may be used to treat spinal cord injuries and retinal implants [127,149]. Axonal directing devices have not been studied in the treatment of CNS injury [116]. This is because polymers coordinate the growth and differentiation of stem cells. Polymers might potentially help repair brain tissue by regulating axonal growth. Cell-to-cell contact is a critical factor in a drug's bioavailability. The design of the scaffold is affected by the transplanted cells [1,150]. There are a few ways to accomplish this. Surface geometry can influence cellular function. Precision neurite growth is made possible by polymers with built-in photonic tuning. The growth of PC12 cells in polyimide micro-channels modifies neurite polarity and length. [6,151]. Microscopic polymers aid infant rat and chick development. The grooves' breadth aids in Schwann cell orientation [7,100]. While astrocytes promote neurite growth, spinal cord neurons maintain neuron survival. Researchers have yet to discover the full extent to which microstructure influences cellular function and growth. There is a correlation between the origin and kind of cell and the active surface detecting responses (for example cell lines, primary cells and stem cells) [86,152]. Neurites allow nerve cells to communicate with other cells and organs. Consequently, it is necessary to analyze the cell and the scaffold separately. Without embryonic stem cells, adult human neural stem/progenitor cells can replace damaged brain cells [111,153]. Adult neural stem cells are found in the SVZ and dentate gyrus of the hippocampal nucleus. Axons can differentiate into neurons, astrocytes, and oligodendrocytes, three cell types essential for brain tissue regeneration [154]. Axonal directing devices have not been studied in the treatment of CNS injury. A polymer scaffold can be used to promote stem cell adhesion, cell and axonal growth in a damaged brain area. These cells and axons can be used to repair damage [8]. The ability of a drug to be implanted is determined by cell contact [9,10].
The transplanted cells have an effect on the design of the scaffold. There are a few ways to accomplish this. Surface geometry can influence cellular function. Precision neurite growth is made possible by polymers with built-in photonic tuning. The growth of PC12 cells in polyimide microchannels has an effect on neurite polarity and determines the size of the resulting neurite [155,156].
Adult human neural stem/progenitor cells can replace brain cells in the absence of embryonic stem cells [157]. Adult neural stem cells are found in the SVZ and dentate gyrus of the hippocampal nucleus. In vitro astrocyte culture employing oligodendrocytes (brain stem cells) [13,14]. Structural microstructure modulates stem cell adhesion, differentiation, neurite outgrowth, and architecture [15,16,158]. Adult stem cell neurite outgrowth is influenced by micropatterning. In addition to regulating neurite outgrowth, researchers may adjust the size of such structures [17]. The following section will discuss the design factors for the neuronal development of stem cells
3.1. Design factors for neural development of stem cells
Making viable biomaterials for use in medicine and the environment requires a solid understanding of stem cell biology and the techniques that drive neural progeny differentiation [22,159]. Researchers created materials that resembled important components of a stem cell niche, or the environment in which these cells exist, to better understand how these components impact a stem cell's decision to differentiate, proliferate, or remain dormant [23,160].
3.1.1. Soluble cues
A population's ancestry can be traced back in part because to soluble morphogens. There is a plethora of research on morphogen cues and spatial gradients [161]. Differentiation of adult stem cells requires multiple developmental signals. Resolvable stimuli are necessary for the development of neuronal biomaterials [25,117]. In the neural tube, each of the four FGF and Wnt creates gradients. Progenitor cell domains give rise to regional brain subtypes. This complex patterning expands the CNS's cellular diversity and specialization [24]. The Shh and BMP/Wnt gradients define the dorsal/ventral axis. A battle rages between BMPs and Wnts on the roof plate and Shh on the notochord and floor plate [25,66]. FGF and Wnt are responsible for the development of neuron axons and dendrites (RA). However, RA and Wnt gradients may also delineate the caudal spinal cord and brain progenitor areas for motor neurons [54,162]. It may be necessary to utilize morphogenic medicines at the right timing and dose to identify brain progenitor cells. As adults, morphogens hold on to their adult niches. BMP signaling is inhibited by the epigenetic noggin [87,163,51]. They boost neurogenesis while suppressing Wnt signaling, say researchers (sFRP3). Concentrating these elements has several effects. As a result, Shh signaling reduces SVZ stem cell availability. NSCN-derived growth factors [164,165]. Their secretions of vascular endothelial growth factor and neurotrophin-3 contribute to the survival of neural stem cells (NT-3). Inflammatory cytokine (IL)−1 beta and interleukin (IL)−6 (IL-6) are produced by astrocytes, and these transmitters regulate NSCs. [118]. GABA, for example, inhibits NSC growth via negative feedback. Extracellular linkages between NSCs and their surroundings influence their fate choices (niche cells) [[166], [167], [168]]. NSC proliferation and differentiation are regulated by Notch and Eph-Ephrin. NSCs are vital in maintaining tissue homeostasis. For NSC differentiation, several criteria must be addressed [169,170].
3.1.2. Extracellular matrix cues
Structural and functional proteoglycans and fibrillar proteins make up the ECM of the nervous system. Many areas of the CNS have distinctive ECMs due to their distinct anatomical and physiological histories. These proteins represent only a small fraction of those discovered in the human body [171,109]. Stem cells produce enough sulfated proteoglycans to boost EGF and FGF-2 production. It is found in the hippocampus and expressed by RGCs and neuronal stellate cells (NSCs) [3,172]. Tenascin-C deficiency reduces NSC proliferation [173]. Less fibrous proteins (collagen and elastins) and sticky glycoproteins (hyaluronan) than other tissues (laminin, fibronectin). Adult neurons are surrounded in a thick pericellular matrix that assists in soma and dendrite growth [135,174]. The active components are tenascin R and hyaluronan PNN [175]. Scaffolds and hydrogels can be made entirely of ECM components or modified to include ECM proteins or peptide derivatives [73,176]. A complex tissue replication may be created by modifying the extracellular matrix to produce stem cell and neuronal niches [88,119].
3.2. Engineering by hydrogel design
The CNS has a distinct ECM structure, as well as other unique features, such as softness. The elastic modulus of brain tissue is 500 Pa, while muscle and bone are 104–109 Pa [16,17]. The extracellular matrix's mechanical, structural, and chemical qualities affect the activity of cellular structures and organelles [177,178]. CNS cells lack weights and hence cannot receive mechanical impulses. The CNS ECM is an engineering wonder. Proteoglycans are plentiful between neurons and glial cells in the CNS but not in other organs [179]. CNS proteoglycans have growth factor binding sites and chondroitin sulfate side chains. Other proteins include serine proteases and MMPs (MMPs) [180]. The perineuronal net surrounds the brain's neurons with proteins and lecticans (PNN). Tenascin-C and Tenascin-R, with hyaluronic acid, make up this compound. Terminally differentiated neurons require homeostasis [181,78]. This ECM helps maintain these functions. Hydrogel devices having biochemicaland mechanical properties similar to the CNS have gotten a lot of research attention [182]. Natural hydrogels include polyacrylamide, polyethylene glycol, and cellulose acetate gels, among others. The hydrogel's rigidity and structure affect cell survival, adhesion, neurite production, and migration [183]. The production of a hydrogel for this purpose involves many considerations. A regenerative hydrogel preserves neuronal cells. To develop cells and neurites, the hydrogel must be microporous [111,153,184]. To provide the cells the right mechanical input, the hydrogel's mechanical properties must match those of natural CNS tissue [9,10]. Hydrogels can create a three-dimensional stem cell niche in vivo, potentially improving stem cell survival and proliferation [14,21]. The following section will give an overview about the remyelination ability of neural stem cells. The following section will discuss the ethical aspects of the use of neural stem cells. Table 4 lists the clinical trials involved with the neural stem cells in the treatment of multiple sclerosis.
Table 4. Clinical trials of neural stem cell therapy in multiple sclerosis.
| SL No. | Clinical Trails Identifier | Study title | Status | Sponsor | Conditions | Enrollment | Interventions | Location |
|---|---|---|---|---|---|---|---|---|
| 1. | NCT03269071 | Neural stem transplantation in multiple sclerosis treatment | Completed | IRCCS San Raffaele | Progressive multiple sclerosis | 4 participants |
|
|
| 2. | NCT03282760 | Safety study of human neural stem cells injections for secondary progressive multiple sclerosis patients | Completed | Casa Sollievo della Sofferenza IRCCS | Secondary progressive multiple sclerosis | 24 participants |
|
|
| 3. | NCT03355365 |
Intrathecal administration of autologous mesenchymal stem cell derived neural progenitors (MSC NP) in progressive multiple sclerosis NP) in progressive multiple sclerosis |
Active, not recruiting | Tisch Multiple Sclerosis Research Center of New York | Multiple sclerosis | 50 participants |
|
|
| 4. | NCT01933802 |
Intrathecal administration of autologous mesenchymal stem cell derived neural progenitors (MSC NP) in patients with multiple sclerosis NP) in patients with multiple sclerosis |
Completed | Tisch Multiple Sclerosis Research Center of New York | Multiple sclerosis | 20 participants |
|
|
| 5. | NCT03822858 |
Expanded access to intrathecal administration of autologous mesenchymal stem cell derived neural progenitors (MSC NP) in progressive multiple sclerosis NP) in progressive multiple sclerosis |
Temporarily not available | Tisch Multiple Sclerosis Research Center of New York | Multiple sclerosis | Not provided |
|
|
| 6. | NCT04121468 | A phase I double blind study of metformin acting on endogenous neural progenitor cells in children with multiple sclerosis | Recruiting | The Hospital for Sick Children | Multiple sclerosis | 30 participants |
|
|
4. Ethical aspects of the use of NSC in MS treatment
The UN Declaration on Universal Human Rights needs a deontological standard and universal principles to succeed (not unique to medicine) [47,54]. Parallel to the field's expansion, biological ethics is becoming increasingly explicit in its connection to life science and human existence [185]. In interpersonal relationships, Western culture supports the usage of basic human behavior ideas. In this way, non-medical activities may be restricted [5,95].
Neuroethics has grown in popularity in recent years as the field of neurosciences has grown in demand for classical ethical concerns [22,111,109]. It became necessary to establish more focused study lines guided by professionals in both fundamental and clinical neuroscience because of the vast amount of data and research available [124,186]. Neuroethics promotes the view of neuroscience as a research object in all its forms, rather than a single organ [78,187].
It presently focuses on two aspects of stem cell research: genesis and collection, evaluation, maintenance, and storage [188,52]. The transplantation process itself, complications and outcomes, as well as public health implications and future possibilities, must all be examined. The scientific community continues to debate the distinction between zygote, embryo, and fetus, as well as the moment when life begins [[189], [190], [191]]. Though increasingly popular, little research has been done on the ethical issues raised by using stem cells to treat neurological diseases [83,81].
The following three requirements necessitate rapid promotion and support for adult neural stem cell use: A paradigm shift is required to better understand how mature cells function in recipient tissues [115,133,79]. Bioethical research should continue to consider the well-being of participants, especially those who are ill or at risk of illness [11,156,192]. Innovative research concepts that protect people and reduce risk are deemed beneficial because they advance scientific knowledge, improve public health, and improve medical care [193,194]. This research must also follow all applicable legal and moral norms that comprise a social framework [64,61,195].
Adult brain stem cell transplants are held to the same ethical criteria as other transplants, constituting a bioethical triangle [99,196,108]. Donors of tissue and cells can do so in the donation queue on one side of the hospital. Aside from that, there are no special risks to the donor's life or health, and no potential conflicts of interest [98,74,197]. Medical choices should consider scientific understanding when a condition may be avoided or treated [142,143].
The recipient line is believed to be the major prospective beneficiary, with the same rights (including informed permission), ethical selection and assignment processes, and clear expectations surrounding the process [95,96].
It is important to remember that what is technically viable must also be ethically acceptable. The team includes people involved in scientific research, cell harvesting and transplantation, and follow-up and monitoring [2,5]. The whole transplant team must behave themselves with dignity and respect for the team as a whole [79,198]. It currently recognizes the ideas of truthfulness, justice, equality, autonomy, welfare and confidentiality [4,133].
Concerns about the ethical implications of these medicines and research have led neuroethicists to educate neurologists and other interested professionals [57,116,199]. That new technology does not boost our capacity to run more trials while respecting human life and using ethical and socially acceptable procedures [1,200]. Legal issues as well as eventual availability of such therapy as a routine treatment for specific illnesses were addressed in this scenario. As a remedy, it has been suggested that restrictive patents be banned in this business [6,7,151].
5. Discussion
The transplantation of NSCs has been proposed as a potential treatment for a variety of neurological disorders. However, there are major obstacles to utilizing human NSCs for therapeutic purposes. To far, little is known about the biology of neural stem cells (NSCs), which may provide a source of uncommitted, transplant-ready cells. Understanding the cellular and molecular pathways involved in reducing NSC pathogenicity is essential. Second, very few research efforts have focused on isolating the brain of the receiver from its host's environment. Scientists are interested in the effects of growth hormones, neurotransmitters, and cytokines on NSCs after they have been transplanted. Since NSC transplantation is more successful when the host's microenvironment is changed, this may translate into improved treatment strategies in the future. The identification and characterization of stem cells is further confounded by the fact that they arise at different stages of embryonic development and express different sets of genes. NEP, RG, and adult NSC have not been shown to be related. To be clear, the quality of the injected cells has a significant impact on the success of NSC-based treatment. These results raise the possibility that the intrinsic state of the grafted cell at the time of transplantation is just as crucial as the host milieu in determining cell fate. When it comes to cell replacement, brain progenitors with precise spatial specialization may be more effective than a wide cell population. The outcome of stem cell therapy is affected by a number of factors, including the microenvironment of the host, the origin of the stem cells, the cells' stage of development, and how receptive they are to therapy. There needs to be research into the potential dangers and benefits of treating a tumor that develops after a transplant.
The field of stem cell therapy and research stands to benefit greatly from brain stem cell technologies in the future. Scientists claim this will aid their investigation into the disease's biological mechanisms, which will in turn lead to better treatments and longer, healthier lives for patients. No matter how many human investigations were supposed to be conducted, not a single one turned out to be successful. New research has demonstrated that MSC-derived neurons are uncommon and therapeutically irrelevant, according to experts.
Research into the origins of MS and the transplantation of NSCs in animal models are just two of the many considerations that must be taken into account while establishing a cell treatment based on NSCs for MS. Immune cell-induced demyelination, oligodendrocyte loss, and spinal cord nerve cell deterioration are all factors in the onset of multiple sclerosis. There is no age range or racial group immune to its effects. Drugs that reduce inflammation, promote tissue repair, and safeguard nerve cells were all seen as crucial. In order for an NSC transplant to be successful, the cells must be able to differentiate into osteoproliferative ones (OPCs). It is essential to boost OPCs' maturation capacity to ensure their continued development throughout chronic illness periods. Scientists think NSCs and NPCs can suppress the immune system and boost the body's own NSC supply simultaneously. NSCs will need to modify their behavior if they are to improve the function of microglial cells in the central nervous system. Nevertheless, microglial activation varies with MS stage, therefore the length of treatment must be carefully evaluated. To determine the full scope of cell replacement, more study is required. The next step is to address certain pressing issues. Somatic cells must be turned into iNSCs in sufficient quantities before they can be used to create NSCs or NPCs from human iPSCs. In order to put NSCs produced from hPSCs to medical use in the future, thorough characterization and selection of NSC types are required (hiPSCs). How and when NSCs are introduced into a patient significantly affects the outcome of the transplanted cells and the therapeutic procedures that follow. Genetically engineered neural stem cells (NSCs) are more likely to survive and adapt to their new surroundings. Extensive research on exogenous NSCs in many animal models is recommended by the National Institutes of Health before they are used in human therapeutic trials. It's also a promising concept to assist one's own stem cells in growing, migrating, and differentiating in spite of the obstructions caused by lesions. Without altering the genetic makeup of the mice, this is not conceivable.
6. Conclusion
Although engineered neural stem cells may be advantageous for individuals with progressive multiple sclerosis, few clinical studies support this hypothesis. Despite researchers' best efforts, a substantial amount of work remains. It has long been believed that a lack of suitable cells is a key obstacle to translating preclinical discoveries into clinical trials. Significant advances in the generation of induced stem cells during the past 10–15 years have enabled the development of a vast array of innovative cancer treatments. Before neural stem cells may be used to treat patients, a number of obstacles must be overcome. Examples include manufacturing, quality control, and cost management. For instance, a single autologous iPSC therapy is anticipated to approach $1 million. In contrast, greater manufacturing capacity and efficiency should result in price reductions. To ensure that meaningful results are obtained while adhering to established safety and ethical norms, clinical research must be directed by regulatory bodies and pertinent international organizations. People advocating for neural stem cell transplantation should always put the patient's needs first.